New Drug Designations - June 2023
Shots:
-
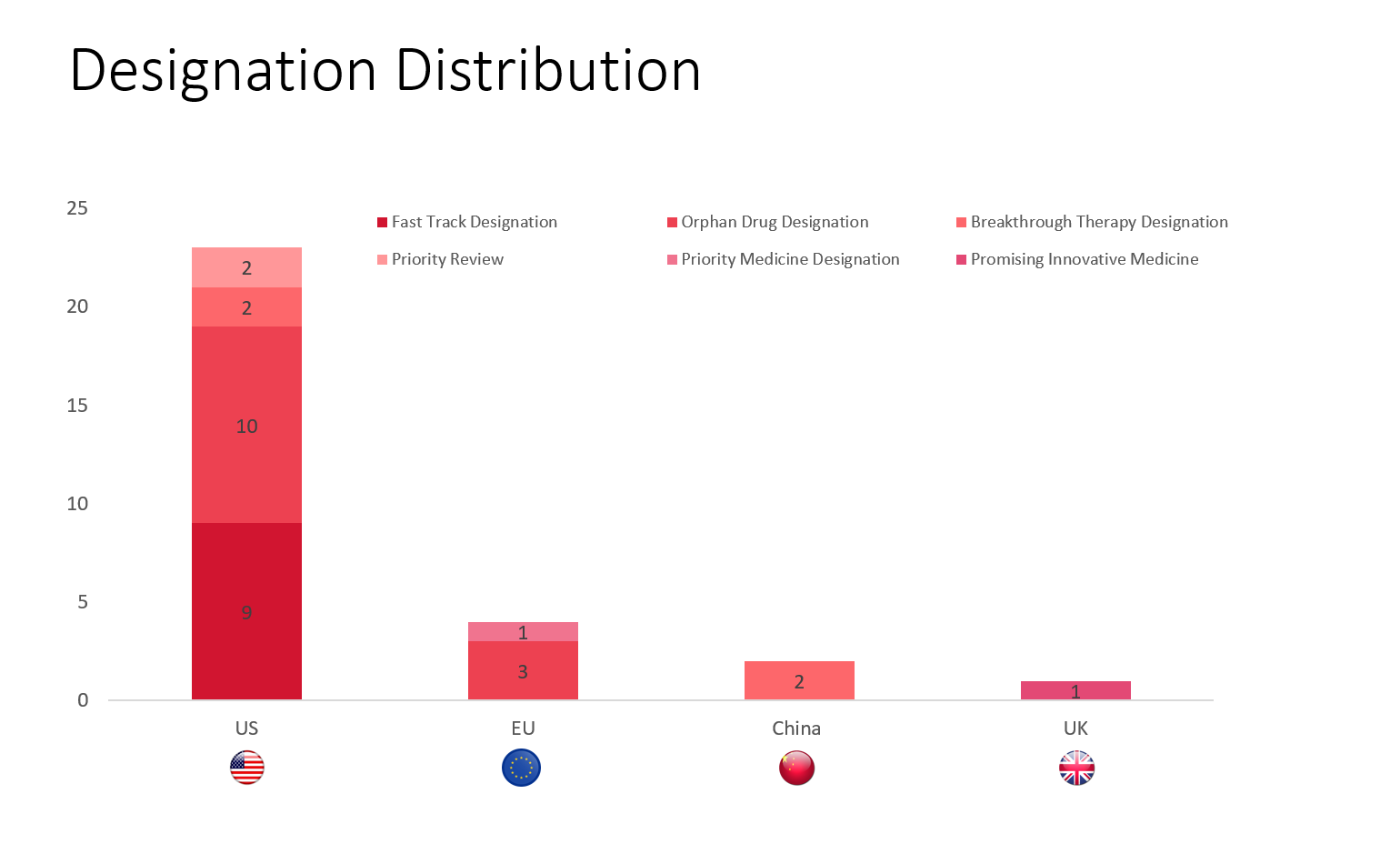
PharmaShots' designation report provides a condensed overview of several drugs and their designations by the US FDA, the EU, China, and the UK. This month’s report includes 7 biological drugs, 13 small molecules and 9 cell and gene therapies
-
CellCentric’s Inobrodib, for multiple myeloma and Rocket Pharmaceuticals’ RP-A601, for PKP2-arrhythmogenic cardiomyopathy were the two drugs to receive both FTD and ODD from the US FDA
-
PharmaShots has compiled a list of a total of 29 drugs awarded with designations by multiple regulatory bodies in Jun 2023

Presendin (Sustained Release Exenatide)

-
Presendin (qw, sub-cutaneous SR) is being evaluated in a randomised, double-blind, PBO-controlled P-III (IIH EVOLVE) study enrolling 240 IIH patients worldwide to reduce ICP in several conditions associated with elevated ICP, incl. moderate and severe TBI
-
Peptron originally developed Presendin (qw, SC, SR) exenatide microsphere formulation.
-
Invex entered into an exclusive collaboration, manufacturing and supply agreement with Peptron in Sep 2021 for Presendin in IIH for all major markets, except South Korea
Inobrodib (CCS1477)

-
Inobrodib (CCS1477) is being evaluated in an ongoing study (NCT04068597) as monotx. and in combination with SoC treatments (pomalidomide and dexamethasone) in patients with r/r multiple myeloma after four or more prior lines of therapy, non-Hodgkin lymphoma, acute myeloid leukaemia or high-risk myelodysplastic syndrome (MDS)
-
Inobrodib, that is administered as an oral capsule and can be used at home without intensive monitoring
Evorpacept

-
Evorpacept showed consistent anti-tumor activity and is being evaluated in a randomized P-II study (ASPEN-06) in combination with trastuzumab, paclitaxel and Cyramza (ramucirumab) for the treatment of HER2+ gastric cancer. The data is anticipated in H2’23
-
Evorpacept (a next-generation CD47 blocker) also received ODD by the US FDA in Jan 2022
VCN-01

-
VCN-01 received ODD for the treatment of retinoblastoma. Currently, it is being evaluated in the P-IIb study (VIRAGE) in combination with SoC CT (gemcitabine/nab-paclitaxel) as a 1L therapy for patients with pancreatic ductal adenocarcinoma (PDAC)
-
VCN-01 systemic, selective, stroma-degrading oncolytic adenovirus that replicates within tumor cells and degrade the tumor stroma which serves as a significant physical and immunosuppressive barrier to cancer treatment
AVB-001

-
In Mar'23, Avenge bio received positive feedback from the USFDA on its preclinical and clinical development plans for pleural malignant mesothelioma. The preclinical data were published in Clinical Cancer Research
-
AVB-001 is also being evaluated in P-I/II single-arm, open-label, dose-escalation and expansion trial for its safety, tolerability, PK, PD and preliminary antitumor in patients with high grade cancer of the ovary, primary peritoneum, or fallopian tube
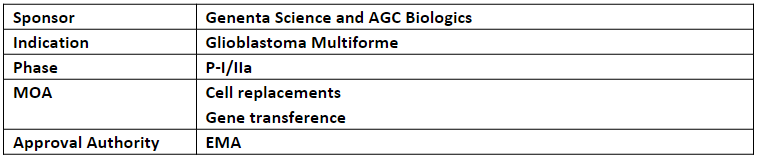
Temferon
-
In Mar’23, Temferon also received ODD from the US FDA for the treatment of GBM
-
Preclinical studies demonstrated that Temferon breaks tumor-induced tolerance, thus allowing the immune system to recognize the tumor and mount a durable immune response
-
Temferon consists of the patient's own stem progenitor cells modified with Genenta's platform to express interferon alpha (IFNα) within solid tumors
KT-253
-
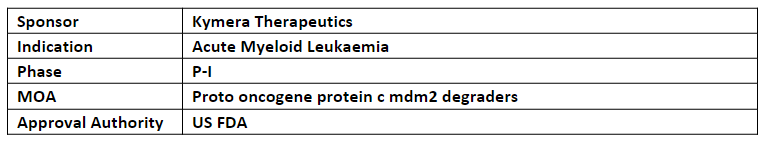
KT-253 (IV, q3w) is being evaluated in the dose escalation P-I trial, initiated in Mar’23, for its safety, tolerability, PK/PD, and clinical activity to treat r/r high grade myeloid malignancies, incl. AML, ALL, lymphoma and solid tumors
-
KT-253 is a potent and selective degrader that targets MDM2, the crucial regulator of the p53
BRG01

-
In Feb’23, BRG01 received IND approval from the US FDA and from China CDE in Dec’22
-
A follow-up application for FTD is being anticipated along with other drugs projected to enter IIT/P-I in 2023 at Singapore, China and the US for the treatment of hepatocellular cancer, gastric cancer and digestive track cancers
ERAS-801

-
ERAS-801 is currently being evaluated as monotx. in P-I study (THUNDERBBOLT-1) to treat recurrent GBM. It is a small molecule EGFR inhibitor that exhibited substantial CNS penetration in preclinical animal studies. P-I data is expected to release in H2’23
-
ERAS-801 has also received FTD by the US FDA in Apr'23 for the treatment of adult patients with GBM with EGFR gene alterations
VCA-894A
-
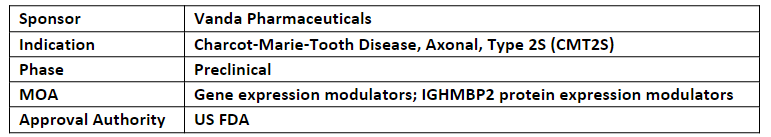
VCA-894A is a novel antisense oligonucleotide (ASO) which specifically targets cryptic splice site variant within immunoglobulin mu-binding protein 2 (IGHMBP2)
-
Mutations in IGHMBP2 expresses CMT2S likely due to alpha-motor neuron loss, and consequently peripheral nervous system deterioration
DTx-1252

-
DTx-1252 showed disease reversal in preclinical rodent models and translation to higher species with IND-enabling studies near completion
-
DTx-1252 is a potential first-in-class FALCON siRNA drug candidate that supresses PMP22 to reverse the effects of CMT1A
FC001
-
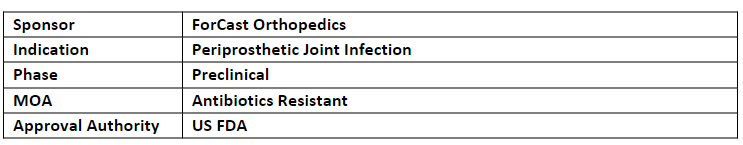
FC001 has also received QIDP by the US FDA for the treatment of PJI
-
FC001 is being developed using ForCast's proprietary technology to deliver a targeted antibiotic therapy directly into the infected joint
RP-A601
-
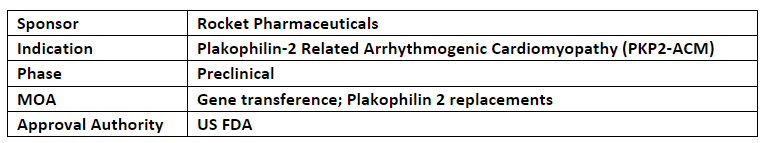
In May’23, the US FDA cleared RP-A601’s IND application to initiate P-I dose escalation study to evaluate the safety and preliminary efficacy of RP-A601 (single dose, starting dose: 8 x 10^13 GC/kg, IV) in at least 6 adult patients with PKP2-ACM
-
The P-I trial will evaluate the impact of RP-A601 (AAV.rh74-based gene therapy) on PKP2 myocardial protein expression, cardiac biomarkers, clinical predictors of life-threatening ventricular arrhythmias, and sudden cardiac death

LUNAR-OTC (ARCT-810)
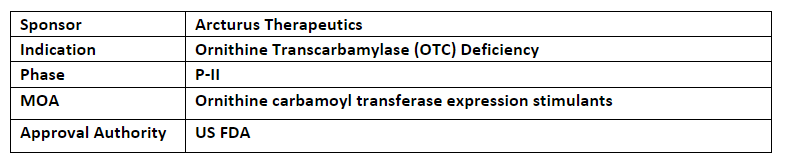
-
ARCT-810, is a mRNA therapeutic candidate designed by using the Arcturus’ extensive and proprietary lipid library and employs LUNAR platform to deliver OTC mRNA to hepatocytes to naturally produce functional OTC enzymes in patients’ own liver cells
Efinopegdutide (MK-6024)

-
At EASL 2023, Merck presented an oral presentation and highlighted the results of a randomized, P-IIa clinical study evaluating the safety and efficacy of efinopegdutide (SC, QW) in adult patients with non-alcoholic fatty liver disease (NAFLD)
-
The P-IIb study of efinopegdutide (MK-6024) for the treatment of NAFLD is anticipated to begin in Jun 2023
-
MK-6024 is being developed under an exclusive licensing agreement between Merck and Hanmi Pharmaceutical for the treatment of patients with NASH
Inobrodib (CCS-1477)

-
Inobrodib (CCS1477) is being evaluated in an ongoing study (NCT04068597) as monotx. and in combination with SoC treatments (pomalidomide and dexamethasone) in patients with r/r multiple myeloma after four or more prior lines of therapy, non-Hodgkin lymphoma, acute myeloid leukaemia/ high-risk myelodysplastic syndrome (MDS)
-
Inobrodib is an oral capsule and can be used at home without intensive monitoring
EP-104IAR
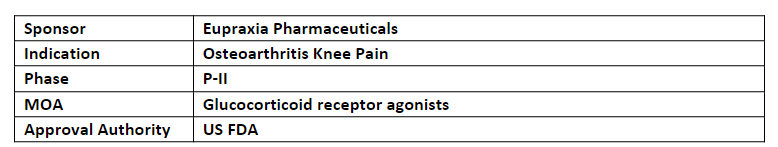
-
The company continues to develop an ongoing P-II study evaluating safety and efficacy of EP-104IAR to treat knee OA. Top-line results for the study are anticipated in Q2’23
-
EP-104IAR encapsulates fluticasone propionate within a microns-thin polymer membrane which is a part of the company’s patented technology platform and has the potential to provide longer pain relief with fewer side effects
ACI-24.060

-
Along with FTD, the US FDA also cleared the IND application to expand the ongoing P-Ib/II trial (ABATE) of ACI-24.060 in patients with Alzheimer's disease (AD) and Down syndrome (DS) in the US. The first DS patient has been dosed
-
All the above-mentioned milestones were based on positive initial interim safety and immunogenicity data from 1st low dose AD cohort while dosing in a 2nd higher dose AD cohort was initiated in 2023
-
Interim safety and immunogenicity data from both cohorts is anticipated in H2’23 and the initial PET data on amyloid plaque reduction in AD is anticipated in H1’24
CAL02
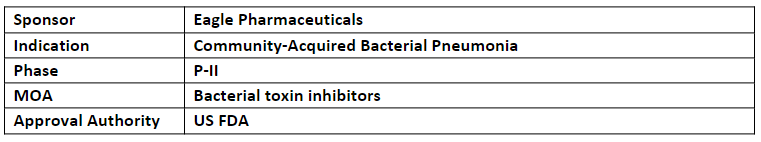
-
Along with FTD, the US FDA also granted QIDP designation (Qualified Infectious Disease Product) under the Generating Antibiotic Incentives Now (GAIN) Act
-
Eagle’s CAL02 also holds the US Patent No.10,744,089 extending its protection until Sep 2035 along with and expects Patent Term Extension for up to 5yrs. until 2040
-
CAL02 (IV) is currently being evaluated safety and efficacy in P-II trial in ~276 patients with SCABP worldwide. The results of P-I study were published in ‘The Lancet Infectious Diseases’
-
CAL02 is a novel first-in-class broad-spectrum anti-virulence agent being developed as an add-on to SoC treatment of SCABP. It is also expected to receive Breakthrough Therapy Designation from the US FDA.
Reqorsa
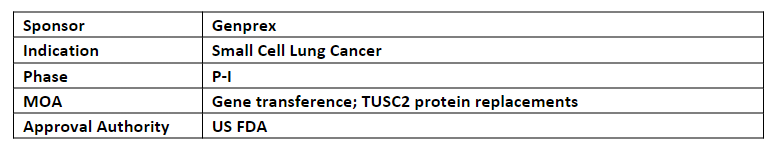
-
Reqorsa + Tecentriq is being evaluated in P-I/II (NCT05703971) dose-escalation trial (Acclaim-3) for the treatment of patients with ES-SCLC who did not develop tumor progression after receiving Tecentriq and CT. First patient enrollment is anticipated in Q3’23
-
P-I portion of the trial is expected to determine MTD in 12 patients and P-II portion of the trial is expected to enroll 50 patients to determine PFS at 18wks.
-
Reqorsa has also received FTDs for:
- Reqorsa + Tagrisso to treat patients with late-stage NSCLC whose disease progressed after treatment with Tagrisso
- Reqorsa + Keytruda to treat patients with late-stage NSCLC whose disease progressed after treatment with Keytruda
KYV-101

-
Kyverna is currently enrolling patients in the P-I study of KYV-101 at multiple sites in the US
-
The company has also filed its first EU CTA for a parallel P-I/II study of KYV-101 in LN to the Paul Ehrlich Institute (PEI) in Germany
-
KYV-101, a novel anti-CD19 CAR T cell therapy designed to deplete B cells, incl. autoreactive B cells, in patients with autoimmune disease in an exclusive worldwide license from NIH to use it in autologous & allogenic as well
-
In a P-I/II study demonstrated anti-lymphoma activity with a significant reduction of cytokines released in patients (n=20) with cancer

Olverembatinib (HQP1351)

-
The P-Ib/II study evaluating olverembatinib demonstrated CBR of 93.8% in patients with the subtype of GIST and data were presented at ASCO 2023 for two consecutive years
-
In Mar’21, olverembatinib received BTD by the CDE to treat patients with CML-CP resistant and/or intolerant to first and second-generation TKIs
-
In Jul’22, NMPA accepted NDA to treat CML-CP and granted Priority Review designation for full approval of olverembatinib
-
Olverembatinib is co-commercialized by Ascentage Pharma and Innovent Biologics in China
Febseltiq (infigratinib)

-
The designation was based on the results from the P-IIa PoC trial (NCT05019794) evaluating infigratinib in 80 patients with FGFR2-amplified LA or metastatic G/GEJ adenocarcinoma patients (57.1%) received 2 prior lines of therapy, 3 prior lines of therapy (33.3%) while 9.5% had ≥3 prior lines of therapy
-
The results showed that pretreated patients experienced an ORR (25.0%, n = 20) & mDoR (3.8mos.) & has the potential to provide clinical benefit in 3L or later gastric cancer
-
The efficacy and safety of infigratinib in this patient population in a P-II study is expected to initiate by 2024 in China
PRGN-2012

-
The designation was based on positive results from P-I study evaluating PRGN-2012
-
The key results from the study incl.
-
Strong response at the RP2D in patients who had an average of 5.8 RRP surgeries (range 3 – 10) in the year prior to PRGN-2012 treatment
-
CR was reported in 50% patients (6 out of 12) requiring no post-treatment surgeries with a minimum follow up of 12mos.
-
PRGN-2012 treatment resulted in a reduction of surgeries in 83% patients (10 out of 12) in the 12mos. following treatment
-
PRGN-2012 was well-tolerated with no DLTs and no TRAEs >gr2
-
-
PRGN-2012 is an investigational AdenoVerse immunotherapy which is currently being evaluated in P-II trial for the treatment of adult patients (n=23) with RRP. The total no. of enrolled patients was increased to 35 at the RP2D
Zenocutuzumab

-
To treat advanced unresectable or metastatic NRG1+ pancreatic cancer following progression with prior systemic therapy or who have no satisfactory alternative treatment options
-
BTD was granted based on the results from the ongoing P-I/II study (eNRGy) and early access program (EAP) evaluating the safety and anti-tumor activity of zenocutuzumab monotx. in NRG1+ cancer
-
As of Jun 2023, >175 NRG1+ cancer patients have been treated with zenocutuzumab monotx. The data is anticipated at a major medical conference in 2023
-
Zenocutuzumab has previously received FTD to treat metastatic solid tumors harboring NRG1 gene fusions that have progressed on SoC therapy in Jan’21 and ODD for the treatment of pancreatic cancer in Jul’20
-
Zenocutuzumab + ADT (enzalutamide or abiraterone) is being evaluated for CRPC and the data is anticipated in H2’23. Zenocutuzumab + afatinib is evaluated for NRG1+ NSCLC

Capivasertib

-
AstraZeneca’s NDA for capivasertib + Faslodex (fulvestrant) has been accepted to treat adult patients with HR+ and HER2- locally advanced or metastatic breast cancer following recurrence or progression on or after an endocrine-based regimen
-
The NDA has been reviewed under Project Orbis and the PDUFA date is during Q4’23
-
The NDA is based on the results from the P-III trial (CAPItello-291) presented at SABCS'22 and published in “The New England Journal of Medicine”
-
The results of the study (CAPItello-291, n=708) demonstrated:
-
40% reduction in the risk of disease progression or death vs PBO + Faslodex in the overall trial population (based on HR: of 0.60, 95% CI 0.51-0.71; p=<0.001; median PFS: 7.2 vs 3.6mos.)
-
OS data were immature at the time of the analysis, early data are encouraging. It is being evaluated as a key 2EP
-
-
Capivasertib also received FTD by the US FDA in Jan’23
Lovo-cel (Lovotibeglogene Autotemcel)

-
In patients of age 12 + yrs. who have a history of vaso-occlusive events (VOEs). PDUFA date for the same is set in Dec 2023
-
Lovo-cel's BLA was based on the efficacy results from 36 patients in P-I/II study (HGB-206) group C cohort with a median 32mos. of follow-up and 2 patients in P-III study (HGB-210) with 18mos. of follow-up each. Safety data was also included from 50 patients
-
Lovo-cel has also received ODD, FTD, RMAT designation, and RPD designation by the US FDA

RP-A501

-
The designation was based on safety and efficacy results of the P-I study which demonstrated consistent and robust improvements in multiple clinical and highly relevant laboratory parameters incl. LAMP-2 protein expression, reduced autophagic vacuoles, brain natriuretic peptide (BNP), high sensitivity troponin I, and left ventricular mass and wall thickness. Improved symptoms, as evaluated by NYHA class and KCCQ-QOL
-
RP-A501 has also received Regenerative Medicine Advanced Therapy (RMAT) designation

Bimzelx (bimekizumab)

- Bimekizumab is a humanized monoclonal IgG1 antibody which is designed to selectively inhibit both interleukin 17A and interleukin 17F
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.